ALCOA (also known as ALCOA plus) is a regulatory framework with a specific set of principles established by the USFDA (United States Food and Drug Administration) to ensure the integrity of the data in the pharma GMP environment is reliable & accurate at any given point in time. Acronym ALCOA was first coined by Mr Stan W. Woollen from the FDA’s Office of Enforcement in the 1990s. Since then, it has become a native term often used today by every person working in the pharmaceutical industry.
Principles of ALCOA
ALCOA principle applies to all areas like research and development, manufacturing, supply chain, testing, etc. Before 2010, the first version of ALCOA principles of data integrity includes the following:
- Attributable
- Legible
- Contemporaneous
- Original
- Accurate
However, in 2010, to amplify the concept of data integrity in pharma, USFDA extended the principles of ALCOA to ALCOA-C, which is also widely known as ALCOA + or ALCOA Plus. These set of new directions are
- Complete
- Consistent
- Enduring
- Available
Now let us try to understand these ALCOA plus principles in pharma with a few practical examples (wherever applicable).

Attributable
The raw data is traceable to the authorized person recording the data. This principle is one of my favourites in ALCOA.
- When documenting any information on a piece of paper, every word written on it needs to be traced back to the person who has recorded it. For example, if a person X is writing an investigation report; all elements written (or) documented on the report shall be traced back to person X. Certain ways to trace back are by a signature or the initials of an individual X and the date it was observed or recorded (Refer below image)

- Suppose person X updated (or) edited the recorded source data. In that case, it needs to be signed (initialed) with the date and an appropriate reason for editing the source data shall be included so that it is traced back to person X (Refer below image). This principle provides complete traceable information to the person who made the changes, when the change was made and why the change was made.

- In the case of electronic data, this information is captured by an audit trail, which is an electronic data capture (EDC) system. This system makes it extremely easy to identify actions like who created, edited, deleted, signed or viewed a document in real-time and a reason for a specific action.
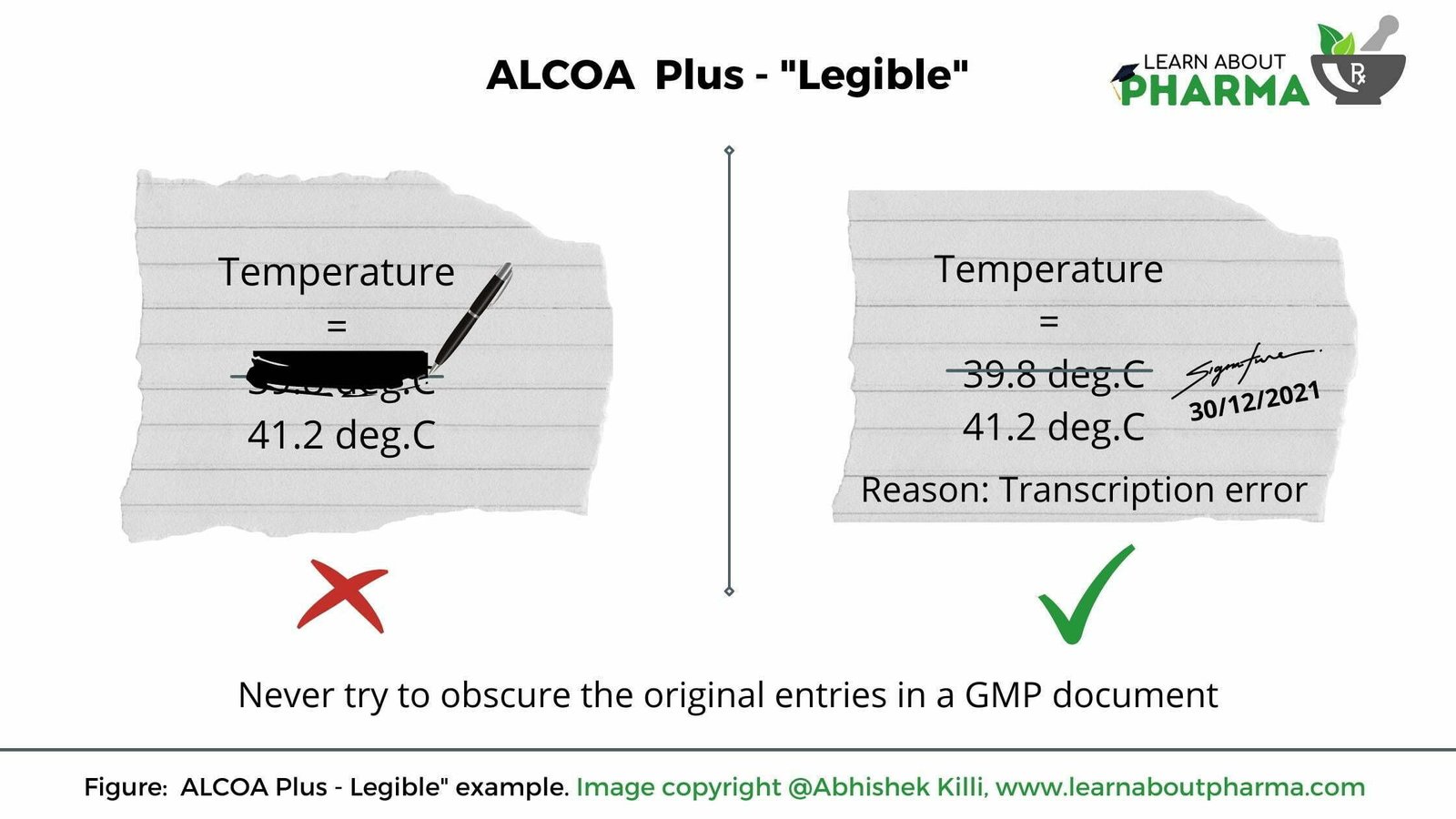
Legible
The raw data is clear and easy to read. Here are a few requirements to record documents.
- Handwriting must be clear. Must make minor or no mistakes and should always try to reduce the number of transcription errors.
- Everything recorded must be easy to read and is attributable, as discussed before.
- Never try to obscure the original entries. Attempts to mask the original entry with a marker, correction fluid or put labels over the data (Refer below image).
When it comes to EDC systems, the problems with Legibility is significantly less because the data is always in a readable and identifiable format.
Contemporaneous
This principle is also one of my personal favourites in ALCOA. The raw data is recorded at the time it is observed. In simple words, data should be recorded when an event occurs.
- Never pre-date or backdate. When any activity is not recorded when made, a chronology should be recorded and documented as “late entry” (Refer below image).
- Forms completed shall not be completed with expected results before execution.

Original
Original means the first-hand data either in paper or electronic medium in which the data is initially recorded. Data is recorded over checklists, records, protocols, reports, spreadsheets or any software application.
- When a pen hits the paper for the first time, this paper document is generally considered a source document in paper form (Refer below image).These source documents shall be archived at a designated place and are referred to whenever required.
- If data is directly entered into the EDC systems, for example, on a notepad with approved steps to follow about a given activity. In that case this electronic notepad file is denoted as a source document.

Accurate
Source data that is complete, truthful, unaltered, free from errors and reflects the actual observation of activity.
- The source data must reflect the true observation. It shall be a thorough representation of facts describing the conduct of the study.
- A detailed justification shall be written and documented accordingly when source documents are incomplete or inconsistent.
- The initial entry shall be struck and corrected if any incorrect data is identified. Initial incorrect entry and the new correct entry shall be visible for future review (Remember principle “Legible”). It is mandatory to sign and put date wherever correction is made to comply with our initial principle “Attributable”
Complete
This is mainly applicable to manual documents. Here the data includes all information from the study. For example, suppose you are recording data related to a given activity. In that case, it should be recorded on a document approved and issued by the quality unit rather than recording it on a piece of paper or any loose sheet.
- All original and subsequent data generated for any activity must be recorded appropriately and retained from moment the documentation is created.
- Deletion or removal of the data must not take place. Necessary measures shall be taken to preserve the data completely.
Consistent
A sequence of events about an activity should align with the expected series of events.
- As discussed before, data from all events shall demonstrate that the data complies with ALCOA.
- Deviation from the expected sequence of events shall be immediately identified and investigated to ensure the quality of the product and process robustness.
Enduring
While we have discussed under principles “Original” and “Complete”, this principle emphasizes to ensure details available for longer durations, even decades, in some situations.
Available
Paper and electronic data are required to be readily available for review, audits, or inspections for the required lifetime of the record. Paper and electronic data should be indexed and appropriately labelled to facilitate retrieval.
In general, data is simply another word for information, which has always been necessary for manufacturing and research. Data drives strategy, measures impact, improves products and processes and finally forms a base for compliance with regulations. Therefore, it is essential to ensure that data is accurate, consistent over its entire life-cycle. Thus, it brings data integrity into the picture, and applying ALCOA Plus principles across all verticals ensures the integrity of data generated in pharma industry.



