Definition:
A pH meter is an instrument used to measure the activity of hydrogen ions in solutions. In other words, it measures the acidity or alkalinity of a solution. It is sometimes called a potentiometric pH meter because it measures the difference in electric potential between a pH electrode and a reference electrode . A detailed pH meter diagram is referred here to give a better understanding for our readers..
- Word pH means Power /Potential (p) of Hydrogen (H); and serves as the most convenient way to measure the relative alkalinity or acidity at a given temperature. The pH rate is directly linked to the potential of both Hydrogen ions (H+) and Hydroxyl ions (OH-) concentrations.
- pH is measured on a scale of 1 to 14 to specify the acidity or basicity of an aqueous solution. The pH of a powerful acid can be less than 0 or greater than 14 for a very powerful base.

- A reading of pH 7 describes the solution as neutral because the activities of both H+ and OH- ions are balanced/equal.
- When the value of pH is less than 7, it means that the activity of H+ ions is greater than that of OH- ions.
- On the contrary, the value of pH increases (higher than 7) when the OH- ions activity in a solution increase at a given temperature.
In search of a better quicker method for testing the pH of solutions, the worlds first modernized electric method was invented by Arnold Orville Beckman – a CALTECH college professor, in 1934, thus paving its way to today’s potentiometric pH meters used commercially.
Working Principle With PH Meter Diagram
We had learned before that the greater the H+ ion concentration, the greater the solution’s acidity. Therefore, an acidic solution with far more hydrogen ion concentration has more significant potential to generate electric current. To explain, it works like a battery that can produce greater voltage, and a pH meter works like a voltameter to measure the solution’s voltage (electric potential). Let us understand the working principle better by following the detailed pH meter diagram below:
- A typical pH meter consists of three different parts: an internal electrode, a reference electrode and a voltameter (Figure – pH meter diagram)
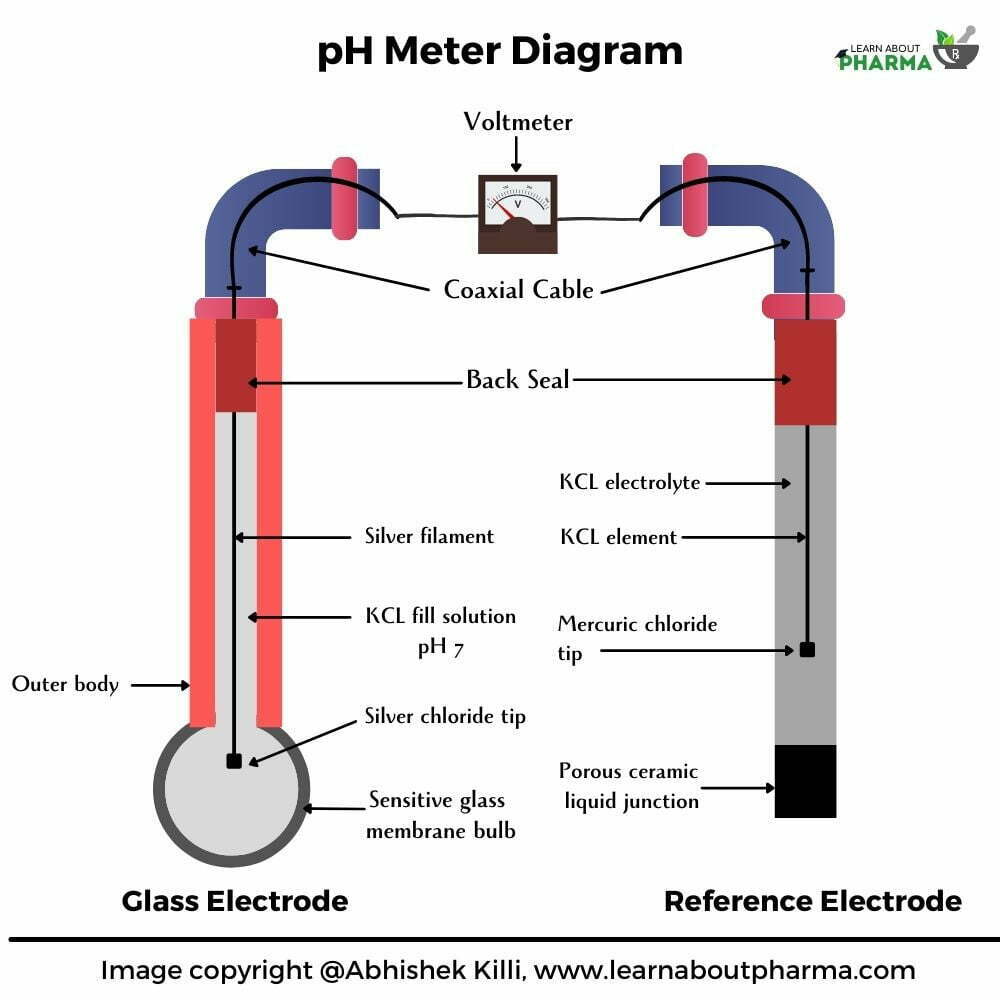

- Practically, when one metal comes in contact with another, a voltage difference occurs due to the differences in electron mobility. This happens the same in the case of two liquids. When two electrodes are dipped in an aqueous solution, the ion-exchange process transpires where the H+ ions in the solution move towards the glass electrode and replace a few metal ions in its special glass coating.
- This way, a small voltage is created, then picked by the glass electrode and passed to the voltameter as shown in the pH meter diagram
- The voltameter then measures the voltage and displays the measurement in pH units on a scale of 0 to 14. Here the reference electrode acts as a reference for the measurement.
- An increase in the voltage means the presence of more H+ ions in the solution and hence more acidic. Similarly, a decrease in the voltage means fewer H+ ions and more OH- ions; therefore, more pH and solution is alkaline.
Types of Electrodes:
- A glass electrode contains potassium chloride (KCL) solution with silver wire coated with silver chloride (AgCl) suspended in it.
- The glass electrode consists of a very special glass coated with silica and metal salts making it even more sensitive to the concentrations of H+ ions surrounding the tip of the bulb.


- The calomel electrode consists of mercury at the bottom of the tube over which a paste of mercury-mercurous chloride is placed. A solution of potassium chloride is placed over the paste. A platinum wire sealed in a hollow tube then helps make the electrical contact.
- This electrode has a non-conductive glass or plastic bulb, which remains unaffected by the surrounding sample solution. The reference calomel solution permanently has a fixed concentration, thus giving a stable voltage
A new generation of pH meters have combination electrodes and are widely used commercially. Here glass electrode (internal) and a calomel electrode (Reference) are both constructed together in a single probe/body and hence called combination (or) combined electrodes.
The glass pH electrode system used nowadays consists of a pH-sensitive measurement glass electrode and a separate reference electrode in potassium chloride (KCl) gel-conducting solution (Figure – combination electrode).
These electrodes are usually housed in the combination sensor, containing both electrodes, which is connected to an electronic meter with a signal amplifier and temperature compensation.

The meter displays the pH reading, which may be uploaded to a computer or controller. A silver wire enclosed in the measurement electrode forwards a signal indicating the difference in acidity between the solutions inside and outside the glass membrane.
The reference electrode has a stable potential, which is independent of the measuring solution and must be calibrated outside the system in a reference solution. The most commonly used reference is a silver/silver chloride electrode in a buffer. The measurement and reference electrodes complete a circuit through the water sample (via a permeable porous junction built in the glass wall, see figure – combination electrode), allowing measurements of the voltage generated by the glass electrode.
Types of pH Meters:
There are three main categories of pH meters commercially used worldwide. They are:
Benchtop pH meters are commonly used in a laboratory set up in the pharmaceutical or food industries. These meters are usually big and measure samples of different sizes and complexities.
Portable pH meters: These meters are pretty handy and easy to carry in a small pocket. They are small in size and are used to measure samples either in a production site or in any place where the sample is to be measured like ponds, lakes, drains etc. These are battery-powered devices that do not last long for longer durations
In-situ pH meters: Also known as pH analyzers, these meters are often integrated with equipment in the manufacturing line to monitor the process continuously. These analyzers are sometimes connected with Electronic Data Capture (EDC) system to collect and document the data (pH readings) in real-time
- pH meters are battery operated or entirely rely on line power ranging from simple, inexpensive devices to complex, expensive lab equipment.
Applications of pH meters:
- It is used to measure the pH of the drug product or raw material sample solutions in the pharmaceutical industry or any chemical plant.
- It is used in Bio-clinical labs to measure the pH of biological fluids like blood, stools, urine, gastric acid, etc.
- They are frequently used in testing soil pH in agriculture to improve crop yield and maximize returns.
- They are often used to test water quality in water treatment plants, pulp and paper industry, swimming pools, rainwater, brewing of wine, oil and gas industries, etc.
- Measure the acidity levels in wastewater in the wastewater treatment plant.
- Measure the exact pH of food-grade products, particularly in the dairy industry ensure safety and quality.
- To measure the exact pH of milk effectively preventing it from turning sour.
While the above list is not exhaustive, it is clear that the pH meter is an essential instrument in the laboratory and has a significant role in product quality testing.
Advantages of pH meters:
- Pocket-sized portable pH meters are easy to carry and measure accurately.
- Lab-scale pH meters are easy to use and occupy less space.
- With proper maintenance and periodic calibration, pH meters can be used for a long time without making them redundant.
- It can be used in a wide variety of protein solutions, viscous preparations and also solutions with strong oxidants and reductants.
Disadvantages of pH meters:
- It needs extra care and attention towards the pH sensing bulb of the electrode and reference junctions from getting dried. It always needs to be stored in a pH storage solution.
- pH meter electrodes quickly tend to clog, get coated with sample and becomes dirty. Cleaning electrodes is a challenge without which the life of the electrodes becomes short.
- The temperature of the solutions always affects the pH readings. Extra attention needs to be taken to ensure the temperature of the solution is at room temperature all time, and also the temperature probe is calibrated before testing the sample for pH.
References:
- G. Orellana, C. Cano-Raya, J. López-Gejo, A.R. Santos, 3.10 – Online Monitoring Sensors, https://doi.org/10.1016/B978-0-444-53199-5.00059-2.(https://www.sciencedirect.com/science/article/pii/B9780444531995000592)